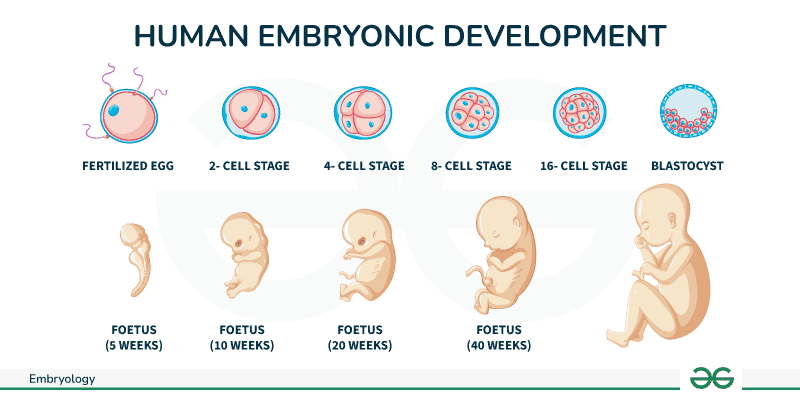
Developmental biology is the field of biology that studies the process by which organisms grow and develop.
Some of the Terms of Developmental Biology:
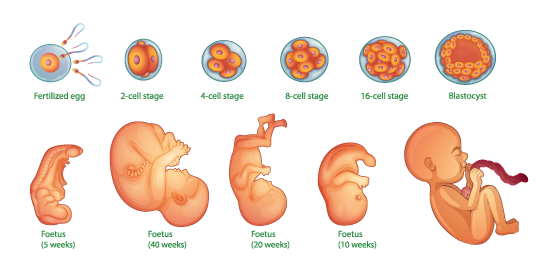
Blastula: The early stage of the embryo, characterized by a hollow sphere of cells.
Blastocoel: The fluid-filled cavity inside the blastula.
Gastrula: The stage following the blastula, during which the three primary germ layers are formed.
Germ Layers: The three layers of cells (ectoderm, mesoderm, and endoderm) that develop during gastrulation and give rise to all tissues and organs in the adult organism.
Ectoderm: The outer layer that forms the skin, brain, and nervous system.
Mesoderm: The middle layer that forms muscles, bones, and the circulatory system.
Endoderm: The inner layer that forms the gut, liver, and other internal organs.
Invagination: The infolding of a region of cells towards the interior of the embryo, initiating gastrulation.
Involution: The inward movement of an expanding outer layer so that it spreads over the internal surface of the remaining external cells.
Blastopore: The opening formed during gastrulation through which cells move inward. It eventually becomes the anus in frogs.
Archenteron: The primitive gut formed during gastrulation as cells move inward.
Dorsal Lip of the Blastopore: The region above the blastopore where cells begin to invaginate; it is crucial for initiating and directing the movements of gastrulation.
Yolk Plug: The mass of yolk-rich cells that is visible at the blastopore during gastrulation.
Spemann-Mangold Organizer (Organizer): A group of cells located at the dorsal lip of the blastopore that orchestrate the development of the embryonic body plan by signaling to surrounding cells.
Epiboly: The movement of epithelial sheets (ectodermal cells) to enclose the deeper layers of the embryo.
Cell Fate Determination: The process by which cells become specialized into specific types during development.
Morphogenetic Movements: The coordinated movements of cells that shape the embryo during gastrulation, including invagination, involution, epiboly, and cell migration.
Blastoderm: The layer of cells formed at the early stage of the embryo, lying atop the yolk.
Area Pellucida: The central, clear area of the blastoderm where cells are less densely packed.
Area Opaca: The peripheral, darker area of the blastoderm where cells are more densely packed.
Epiblast: The upper layer of the blastoderm from which the three germ layers (ectoderm, mesoderm, and endoderm) will develop.
Hypoblast: The lower layer of cells beneath the epiblast, which contributes to extraembryonic structures but not to the embryo itself.
Primitive Streak: A structure that forms along the midline of the epiblast, marking the beginning of gastrulation. Cells move through the primitive streak to form the mesoderm and endoderm.
Primitive Groove: The depression within the primitive streak through which cells migrate inward.
Hensen’s Node (Primitive Node): The anterior (head) end of the primitive streak, functioning as an organizer for gastrulation and future body axis formation.
Koller’s Sickle: A crescent-shaped region at the posterior edge of the area pellucida, involved in the formation of the primitive streak.
Ingression: The movement of individual cells from the epiblast into the interior of the embryo through the primitive streak, contributing to the mesoderm and endoderm layers.
Mesoderm: The middle germ layer formed during gastrulation that will give rise to muscles, bones, and the circulatory system.
Endoderm: The inner germ layer formed during gastrulation that will give rise to the gut, liver, and other internal organs.
Ectoderm: The outer germ layer remaining in the epiblast that will give rise to the skin, brain, and nervous system.
Notochord: A rod-like structure formed from mesodermal cells migrating through Hensen’s node, serving as a precursor to the vertebral column and signaling center for neural development.
Germ Layer Specification: The process by which cells in the epiblast are allocated to become specific germ layers (ectoderm, mesoderm, or endoderm).
Extraembryonic Membranes: Structures that support the developing embryo, formed from cells that migrate through the primitive streak but do not contribute to the embryo itself (e.g., the yolk sac, amnion, chorion, and allantois).
Morphogenetic Movements: The coordinated movements of cells during gastrulation, including ingression, convergent extension, and cell migration.
Convergent Extension: The process by which a tissue layer narrows along one axis and elongates along a perpendicular axis, contributing to the shaping of the germ layers.
Cell Fate Mapping: Techniques used to trace the developmental pathways of individual cells during gastrulation to determine their eventual contributions to the germ layers and embryonic structures.
Neurulation: The process during embryonic development where the neural tube forms, which eventually gives rise to the central nervous system (brain and spinal cord).
Neural Plate: A thickened region of the ectoderm that gives rise to the neural tube.
Neural Tube: The neural tube is a structure in the early embryo that develops into the central nervous system, which includes the brain and spinal cord.
Neural Crest: A group of cells that emerge from the edges of the neural tube and migrate to form various structures, including peripheral nerves, melanocytes, and facial cartilage.
Primary Brain Vesicles: The primary brain vesicles are the initial three bulges at the anterior end of the neural tube that form in the early development of the vertebrate brain.
Prosencephalon (Forebrain): Will subdivide into the telencephalon and diencephalon.
Mesencephalon (Midbrain): Remains undivided.
Rhombencephalon (Hindbrain): Will subdivide into the metencephalon and myelencephalon.
Secondary Brain Vesicles: The further division of primary brain vesicles into five regions:
Telencephalon: Develops into the cerebral hemispheres, cerebral cortex, corpus striatum.
Diencephalon: Develops into the thalamus, hypothalamus, and epithalamus, pituitary, pineal gland.
Mesencephalon: Develops into the midbrain.
Metencephalon: Develops into the pons and cerebellum.
Myelencephalon: Develops into the medulla oblongata.
Neurogenesis: The process by which new neurons are generated from neural stem cells and progenitor cells of the brain.
Gliogenesis: The formation of glial cells from neural stem cells and progenitor cells. Glial cells provide support and protection for neurons.
Radial Glia: Specialized glial cells that serve as scaffolding for migrating neurons during brain development.
Ventricular Zone: The region lining the neural tube where neurogenesis and gliogenesis predominantly occur.
Synaptogenesis: The formation of synapses between neurons as the brain matures.
Myelination: The process by which axons are coated with myelin, a fatty substance that speeds up neural transmission. This is primarily performed by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system.
Cerebral Cortex: The outer layer of the cerebrum, involved in higher brain functions such as sensory perception, cognition, and motor control.
Hippocampus: A brain structure involved in memory formation and spatial navigation.
Neural Induction: The process by which signals from the mesoderm and ectoderm trigger the formation of neural tissue from the ectoderm.
Morphogen Gradients: Gradients of signaling molecules (morphogens) such as Sonic hedgehog (Shh) and bone morphogenetic proteins (BMPs) that pattern the neural tube along the dorsal-ventral axis.
Axon Guidance: The process by which neurons send out axons to reach their correct targets, guided by a combination of attractive and repulsive cues.
Cerebellum: A structure derived from the metencephalon, involved in motor control and coordination.
Thalamus: A structure derived from the diencephalon, serving as a major relay station for sensory information.
Optic Vesicle: Early outpouchings from the diencephalon of the forebrain that will give rise to the eyes.
Lens Placode: A thickened area of the surface ectoderm that will invaginate to form the lens.
Optic Cup: A two-layered structure formed by the invagination of the optic vesicle. The inner layer becomes the neural retina, and the outer layer becomes the retinal pigment epithelium (RPE).
Neural Retina: The inner layer of the optic cup that differentiates into various types of retinal neurons, including photoreceptors, bipolar cells, and ganglion cells.
Retinal Pigment Epithelium (RPE): The outer layer of the optic cup that plays a crucial role in nourishing the retinal cells and absorbing excess light.
Lens Vesicle: The structure formed when the lens placode invaginates and pinches off to become a hollow vesicle, which later differentiates into the lens.
Cornea: The transparent front part of the eye, derived from both the ectoderm and the mesoderm, which allows light to enter the eye.
Iris: The colored part of the eye that controls the diameter of the pupil and, thus, the amount of light reaching the retina.
Pupil: The opening in the center of the iris that regulates the amount of light entering the eye.
Sclera: The tough, white outer layer of the eye that provides structural support and protection.
Choroid: The vascular layer of the eye containing connective tissue, lying between the retina and the sclera, which provides oxygen and nutrients to the eye tissues.
Optic Stalk: The structure connecting the optic vesicle to the forebrain, which will eventually form the optic nerve.
Optic Nerve: The nerve that transmits visual information from the retina to the brain.
Fovea: A small central pit in the retina, densely packed with cone photoreceptors, responsible for sharp central vision (more pronounced in some vertebrates like birds and humans).
Morphogenetic Movements: The coordinated movements of cells and tissues during eye development, including invagination, folding, and differentiation.
Differentiation: The process by which cells become specialized in structure and function. In eye development, this includes the differentiation of lens cells, retinal cells, and other ocular tissues.
Inductive Interactions: Signals from one tissue or cell layer that influence the development of another tissue or layer, crucial for the proper formation of the eye structures. For example, interactions between the optic vesicle and the overlying ectoderm induce the formation of the lens placode.
Photoreceptors: The cells in the retina (rods and cones) that detect light and convert it into electrical signals.
Extracellular Matrix (ECM): A network of proteins and other molecules that provide structural and biochemical support to surrounding cells, playing a critical role in eye development and the maintenance of tissue architecture.
Retinoic Acid: A derivative of vitamin A that acts as a signaling molecule during eye development, influencing cell differentiation and patterning..
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage preimplantation embryo. They have the ability to differentiate into any cell type in the body. Here are some important terms related to ESCs:
Pluripotency: The ability of ESCs to differentiate into all cell types of the three germ layers: ectoderm, mesoderm, and endoderm.
Totipotency: The ability of a cell to give rise to all cell types, including both the embryo and extraembryonic tissues. Zygotes and early blastomeres are totipotent, but ESCs are pluripotent.
Blastocyst: An early-stage embryo consisting of an inner cell mass, which gives rise to ESCs, and an outer layer called the trophoblast, which forms part of the placenta.
Inner Cell Mass (ICM): The group of cells inside the blastocyst that will develop into the entire embryo, from which ESCs are derived.
Differentiation: The process by which ESCs develop into more specialized cell types.
Self-Renewal: The ability of ESCs to proliferate indefinitely while maintaining their undifferentiated state.
Embryoid Bodies (EBs): 3D aggregates of ESCs that can differentiate spontaneously into various cell types representing all three germ layers.
Feeder Layer: A layer of mitotically inactivated cells (often mouse embryonic fibroblasts) used to provide a supportive environment for the growth of ESCs.
Culture Medium: The nutrient-rich solution used to grow ESCs in vitro, often supplemented with factors that maintain pluripotency or induce differentiation.
Teratoma: A type of tumour that arises from pluripotent cells and contains tissues from all three germ layers, used as a test to demonstrate the pluripotency of ESCs.
Induced Pluripotent Stem Cells (iPSCs): Somatic cells reprogrammed to a pluripotent state by introducing specific transcription factors, exhibiting similar properties to ESCs.
Epigenetics: Heritable changes in gene expression that do not involve changes to the DNA sequence, playing a crucial role in maintaining the pluripotent state of ESCs.
Chimera: An organism containing cells derived from more than one zygote. ESCs can be introduced into a developing embryo to create a chimera, demonstrating their developmental potential.